Accelerating Viral Vector Production with Audentes – Webinar Recap
Benchling Empowers Audentes’ Viral Vector Production Team Efficiency
The global gene therapy market continues on an upward trajectory of a 36% CAGR from 2019 through 2026. While this brings exciting new opportunities, outdated lab management tools slow down the ability to bring products to market. But as a San Francisco gene therapy company demonstrated in a recent webinar with Benchling, running your lab with modern software solutions can make all the difference.
Gene Therapy At Center Stage
Audentes Therapeutics develops and commercializes innovative solutions for serious, rare neuromuscular diseases. They are an Adeno-Associated Virus (AAV) based genetic medicines company using viral vector tools. In cell and gene therapy, viral vectors are used to transfer a gene into a target cell, essentially delivering the genetic medicine. Viral vectors are a critical tool in biopharma, and viral vector production is an essential function in cell and gene therapy R&D.
Yet despite developing these advanced medicines, viral vector production and cell and gene therapy R&D overall are hindered by outdated legacy data management systems and technologies in the lab. These inefficiencies are seen across the viral vector production workflow, from plasmid creation to viral production to project analysis and sample handoffs.
Viral Vector Production Team At a Crossroads
The Audentes process development team was experiencing roadblocks to achieving their important work in the lab: error-prone data captured on paper, difficulty finding spreadsheet information, duplicative paperwork, challenges tracing samples with fragmented data sources and other data traceability and management problems.
"For any single experiment, we don't have a centralized place for all of this really important data," Nikita Lim, Process Development Engineer, said in the webinar.
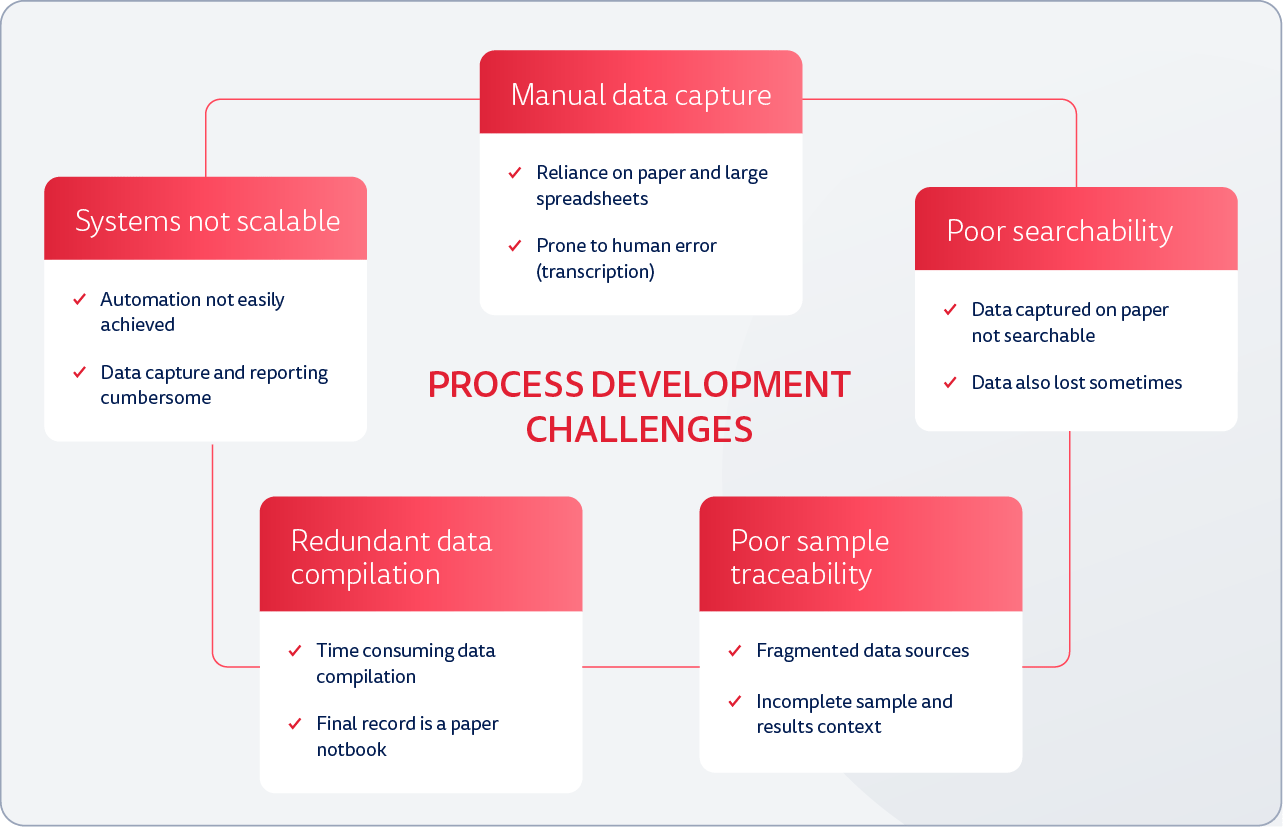
What's more, they needed to scale their work as the group became larger, experiments with bigger data sets became more frequent, and lab responsibilities expanded. Standardizing and capturing data became a critical priority.
Audentes had trialed various solutions in the past, but they were not modular and couldn't expand to teams in the future. Enter Benchling.
Managing Vector Engineering from Design to Production
The Audentes process development team trialed Benchling's unified, cloud-based platform that addresses R&D needs with a suite of applications and services built specifically for the complexities of viral vector production. With a 360° view of R&D innovation, Benchling accelerates, measures, and forecasts the entire vector production workflow from a single source so Audentes can manage everything from discovery to bioprocessing. Benchling's integrated lab notebook, registration, process management, and molecular biology tools made it easier to design, document, and track both the vector engineering process and batches of virions across experiments and roles.
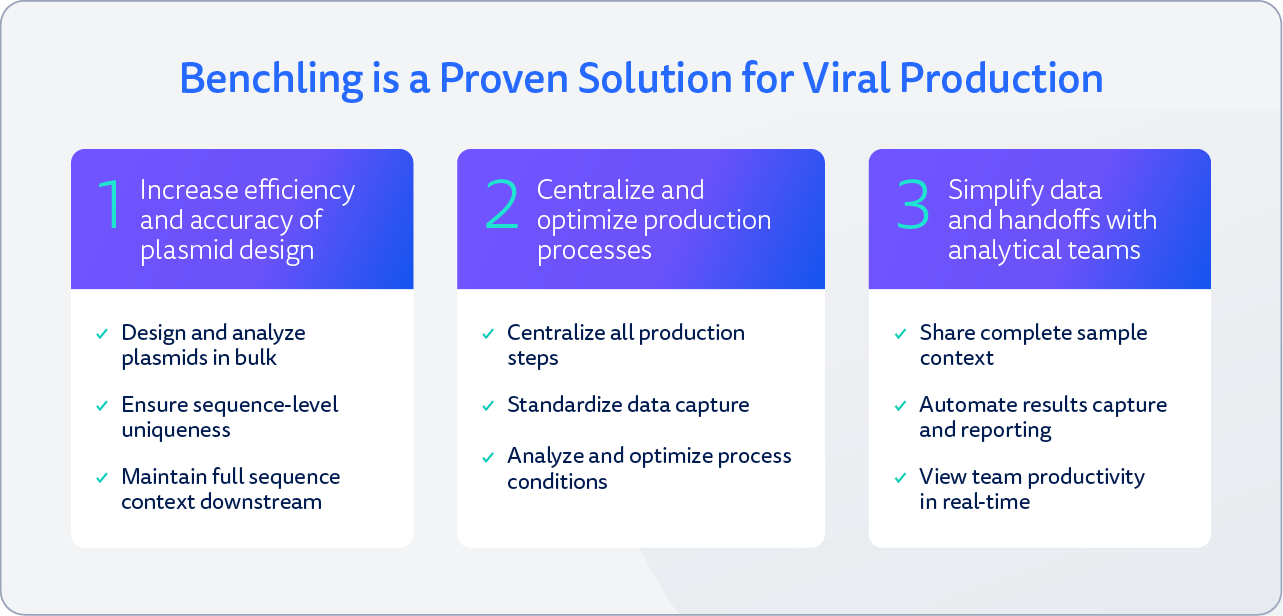
Results: Immediate and Impactful
Benchling's all-in-one solution increased the efficiency and accuracy of plasmid design, centralized and optimized production processes, and streamlined data and sample handoffs with the analytical team.
"Templatizing operations in the lab has reduced operator errors because there is no redundant work…it has saved our team a lot of time and allows us to focus on true development tasks," Lam said. "With Benchling, it definitely allows us to think more about what we can improve on and where the gaps [are]…and troubleshoot our process." Specific benefits included:
"With Benchling, it definitely allows us to think more about what we can improve on and where the gaps [are]…and troubleshoot our process." – Nikita Lam, Process Development Engineer, Audentes Therapeutics
Increased efficiency in lab coordination and enhanced visibility into lab operations, cutting redundant work and operator errors
An increase in routine data collection and reporting productivity, including more compliance, timeliness, and experiment detail in notebooking activities
The capability to scale workflows and processes as they grow
Newfound abilities to share data and other insights in real-time
The Future of Viral Vector Production Is Bright with Benchling
With the process development team's success with Benchling, the company's engineering, manufacturing, and other groups are also interested in trialing this solution. Because of the platform’s modular design and codeless configuration, Audentes can easily customize the system to meet each team's exact requirements.
Audentes Therapeutics is just one example of how cell and gene therapy, viral vector production, and process development can usher in the next generation of data management, control, and efficiency with an elegant, simple software solution.
Powering breakthroughs for over 1,200 biotechnology companies, from startups to Fortune 500s
