
Data sheet





Built-in controls to ensure compliance with GxP workflows, reduce errors, and maintain transparency across experiments.
Use e-signature controls per 21 CFR Part 11 and Annex 11
Track every edit with visible timestamps and a complete audit trail
Control system changes with user access management
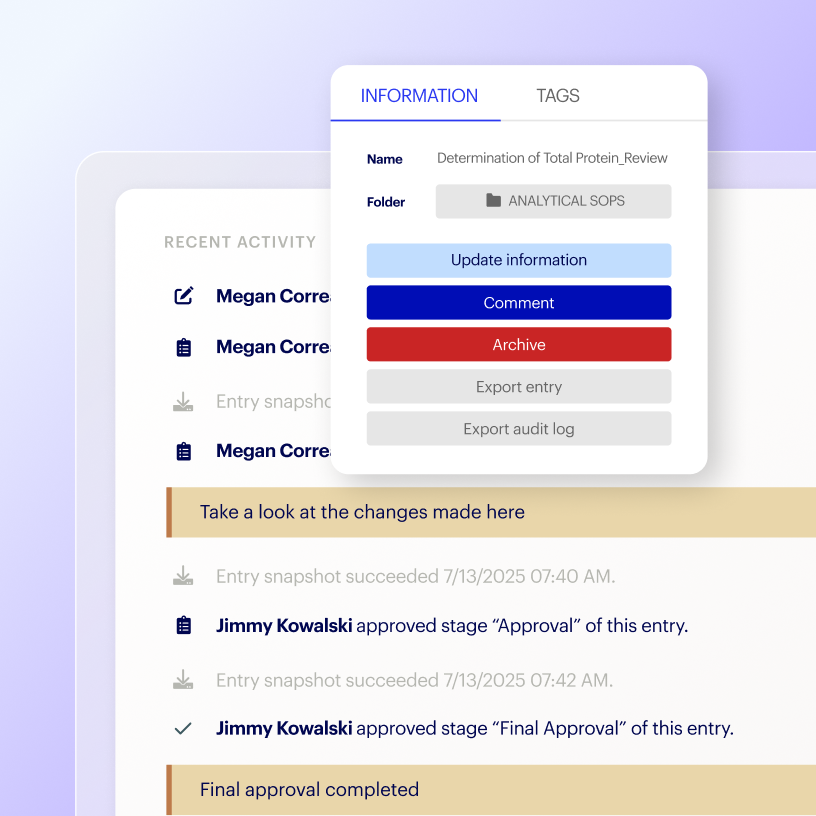
Maintain robust control over GxP workflows, while giving non-GxP research teams the flexibility that they need.
Separate GxP and non-GxP work for clarity and efficiency
Reduce GxP burden on non-GxP research teams
Stay up to date with controlled, quarterly system releases
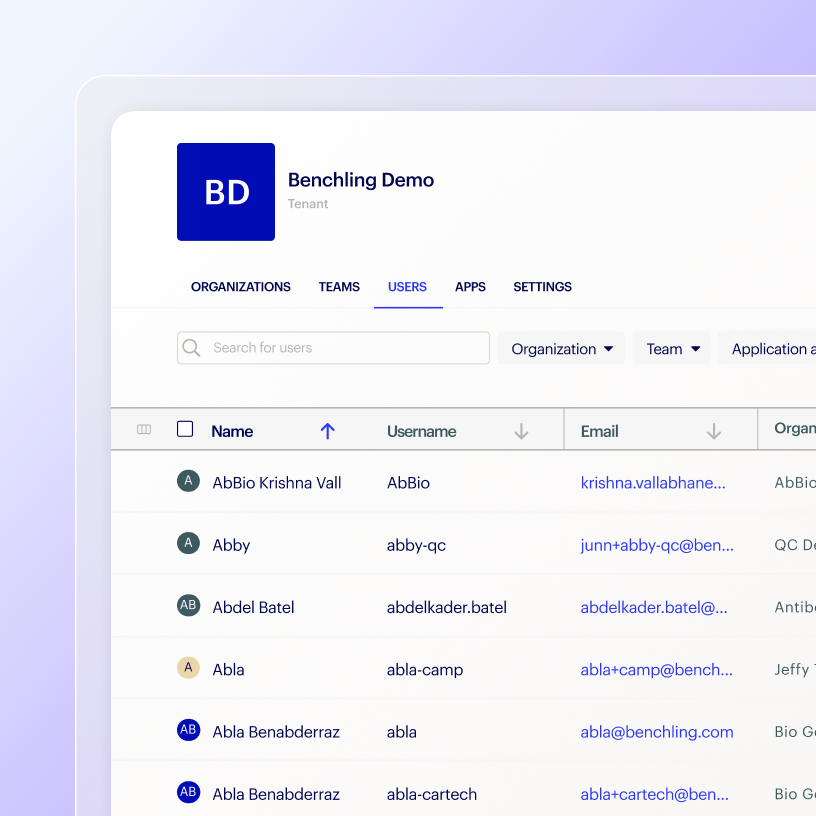
Pass third-party and regulatory audits by complying with the latest regulatory, security, and quality standards.
Stay ready for third-party audits
Comply with ISO 27001 security standards
Align with ISO 9001 QMS guidance
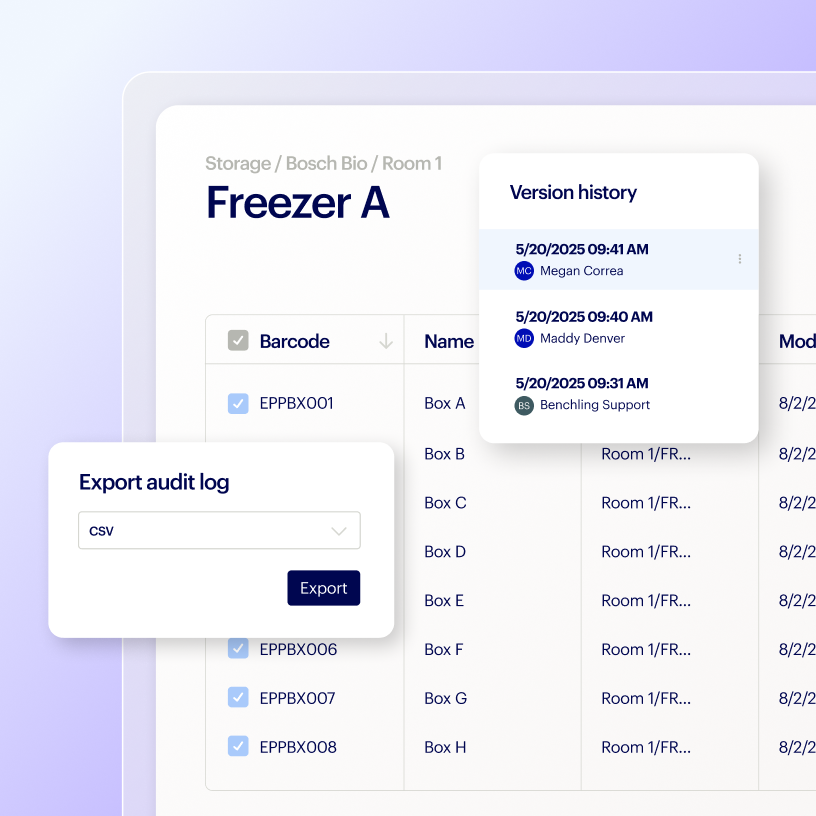

Deploy a dedicated tenant to manage GxP workflows and give teams control to mitigate compliance risks.

Stay compliant with software controls that meet the latest regulatory, security, and quality requirements.

Access expert guidance and documentation for the full validation process, including IQ/OQ, with quarterly change packages and risk assessments.

Capture experimental data from GxP studies in structured templates and results tables that can be queried or searched easily.

Keep consistent sample naming, ontology, and hierarchy across R&D.

Coordinate sample submissions, data handoffs, and results reporting across GxP teams.
Having one R&D data management solution allows us to carry forward insights and workflows into the more structured and controlled development processes, giving us a real competitive advantage.
Quality Control Manager, New England Biolabs