Create Structured R&D Processes with New Template Capabilities
Companies throughout the life science industry are pushing the boundaries of innovation through creative experimentation, out-of-box thinking, and a willingness to go beyond the status quo.
But as products move through R&D, there comes a time in the product innovation life cycle when teams need to standardize processes. This can happen in early research, as companies establish fixed discovery methods, or later downstream, in development and QC — where process optimization and repeatability become vital.
To support this need for standardized processes, we’re excited to introduce new fill-in template functionality that teams can use for everything from designing repeatable experiments and analytical tests to establishing SOPs.
The Need for New Tools to Standardize Processes
Historically, legacy software solutions such as a Lab Information Management Systems (LIMS) haven’t kept pace with rapidly advancing R&D. Built for specific use cases, LIMS lock users into rigid procedures that aren’t extensible over time, or easily transferable across processes or groups. Many organizations have also relied upon separate LIMS systems for different functions: a LIMS for analytical testing, another for process development, and so one. Such fragmentation has made it difficult to maintain accurate, usable data and easily share knowledge.
But in the fast-evolving world of life sciences discovery and development, R&D teams need flexible tools that can support multiple complex procedures. That’s where Benchling’s more structured templates come in.
Creating Repeatable Processes
With fill-in templates, R&D teams can balance configurability with control. Team leads and supervisors can work with Benchling admins to create lockable Notebook entries that define, document, and lock down key processes in order to regulate how tasks are completed.
Easy-to-follow guidelines simplify and speed up work for teams while reducing the potential for inadvertent data modifications. This reduces the time needed to review data, while instilling confidence that groups are accurately entering data and following processes.
Groups across R&D can leverage more controlled fill-in templates to define virtually any process. Below are three common use cases across discovery, analytical testing, and quality.
Discovery: Standardize Experiment Execution to Reduce Data Capture Errors
With structured templates, research teams can bring repeatability to experiments, simplifying study design and enabling the execution of larger-scale process runs.
In the world of T-cell therapies, for example, researchers balance a variety of detailed experiments across multiple phases of cell growth, while simultaneously tracking which scientists interact with cells and for which purposes. Defining and locking down procedures from cell activation to cell freezing centralizes work streams, and maintains data integrity of cells’ attributes as they transfer among cell line development, upstream, downstream, and formulation teams.
With this centralized data, downstream teams can trace the lineage of each sample back to its origin, enabling them to better understand the results of T-cell performance in the clinic. As regulatory teams prepare for IND filings, access to this rich database of high quality sample information enriches a complete and compliant regulatory submission.
Development: Streamline Analytical Testing Procedures
Analytics operations teams sit at the center of many different development workflows, ingesting, testing, and tracking results data across touch points. For large molecule assays in particular, teams carry out multiple in-depth tests at different times, generating large datasets and numerous samples — all of which need to be tracked and managed.
These templates created for analytical tests simplify this tracking and management work by keeping teams aligned to standard processes for assay data capture and protocol execution. Operations leads — who own, create, and update these templates — centralize these protocols and establish a uniform model for results entry.
They can even lock down specific formulas within the templates to eliminate computing errors or accidental formula changes. This granularity is crucial for process teams, who rely on high-quality, trackable data to understand the full lineage of their samples.
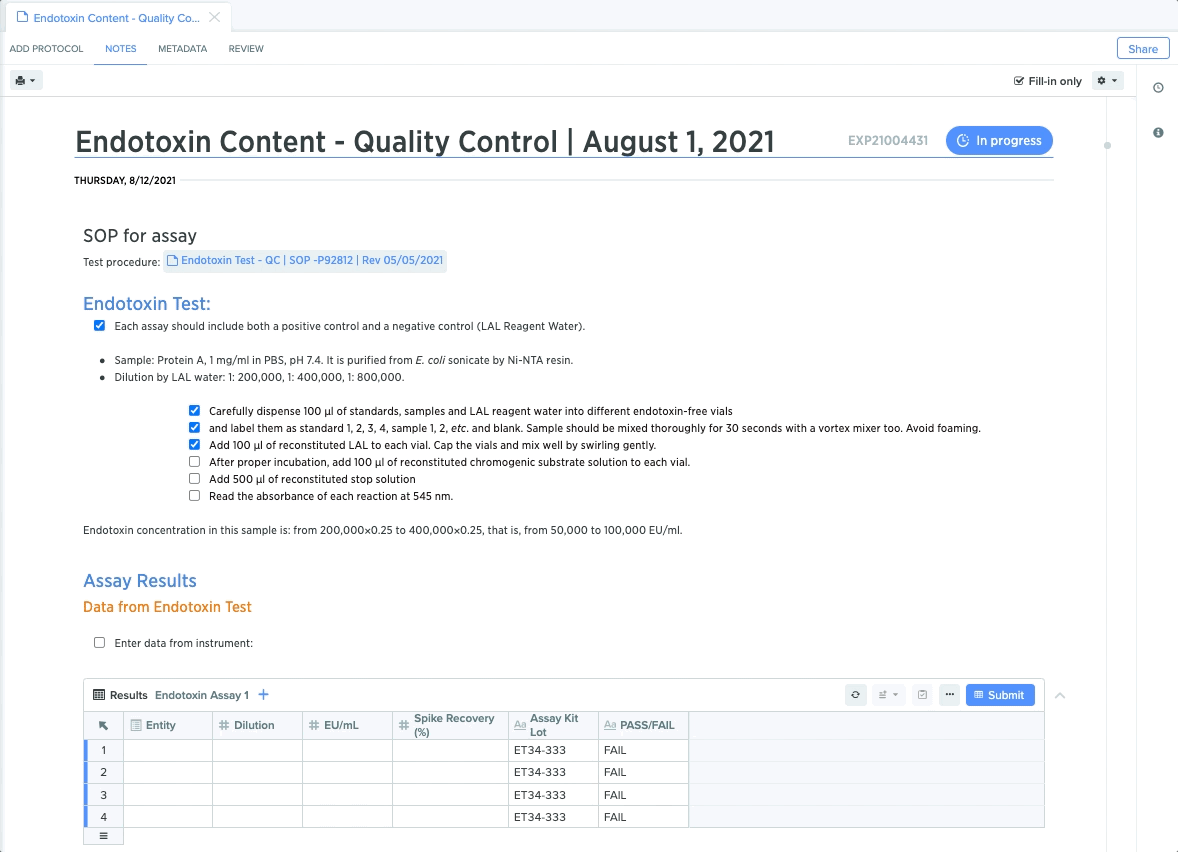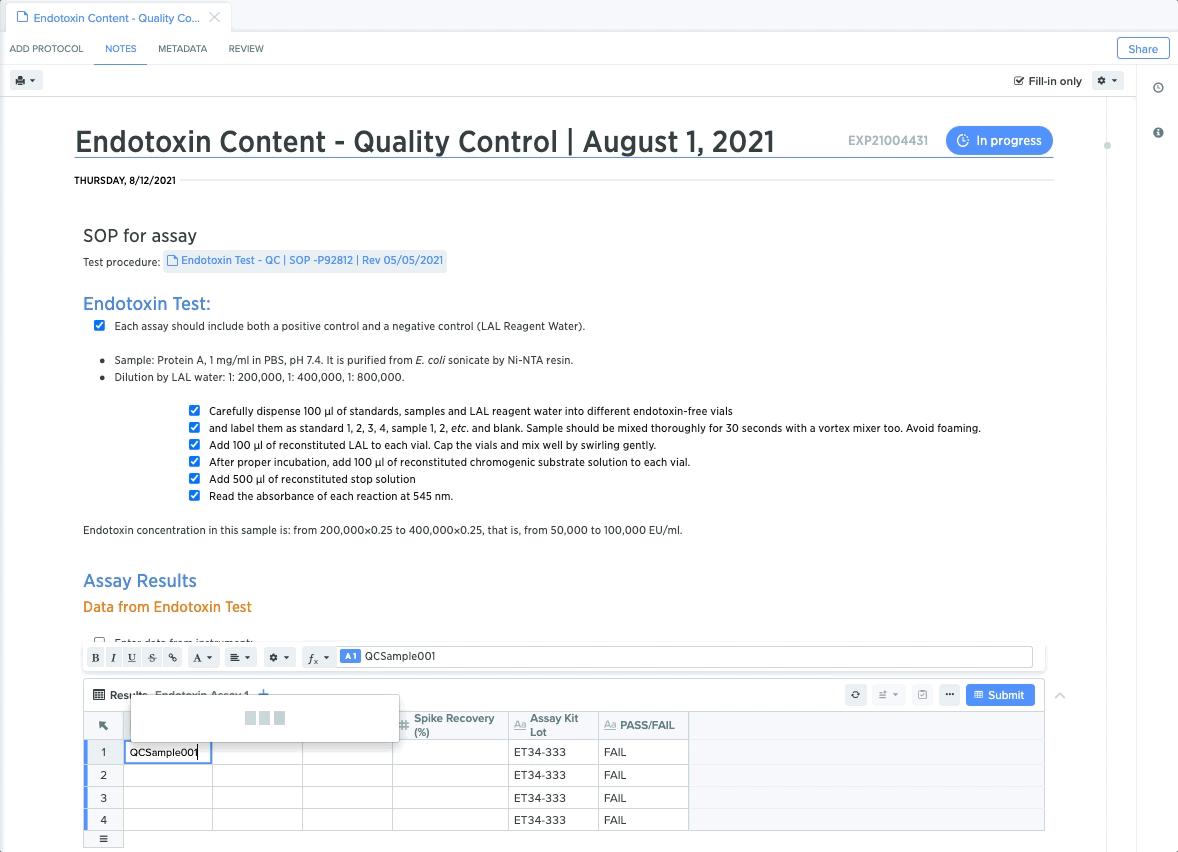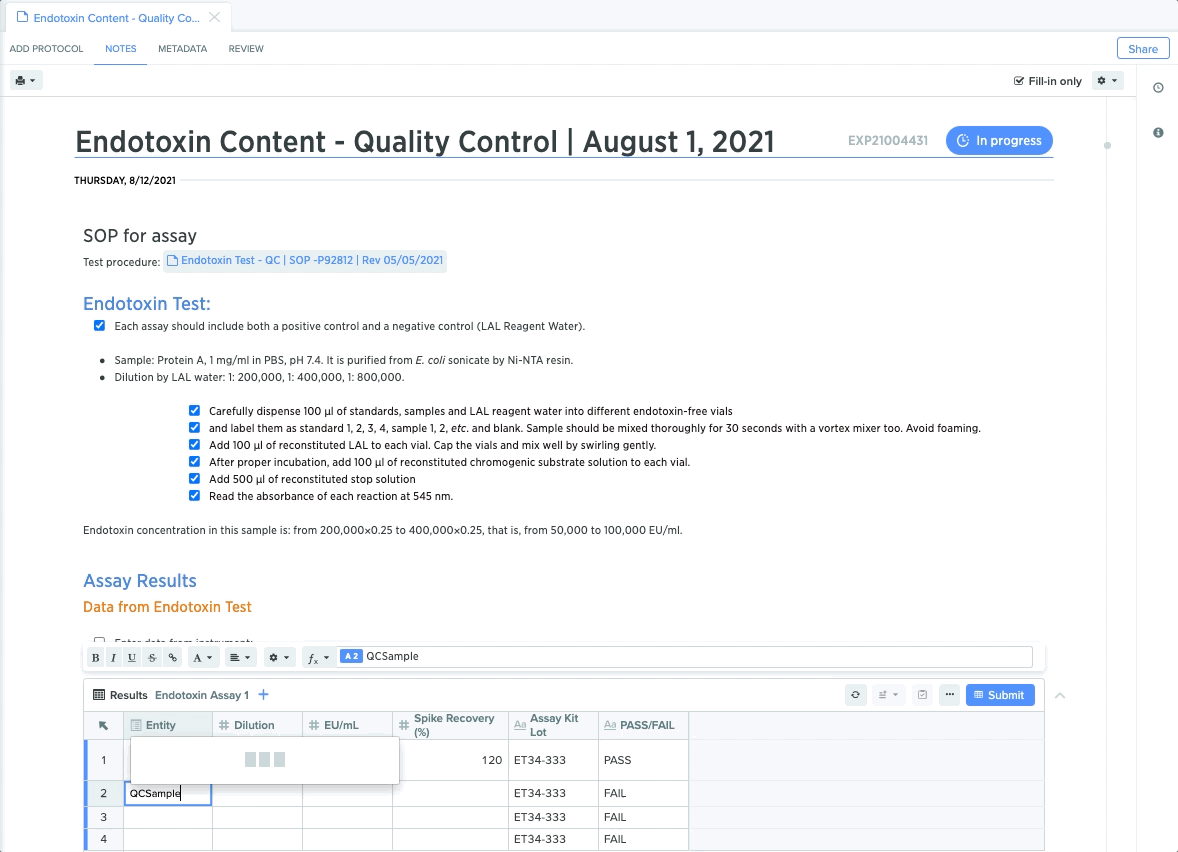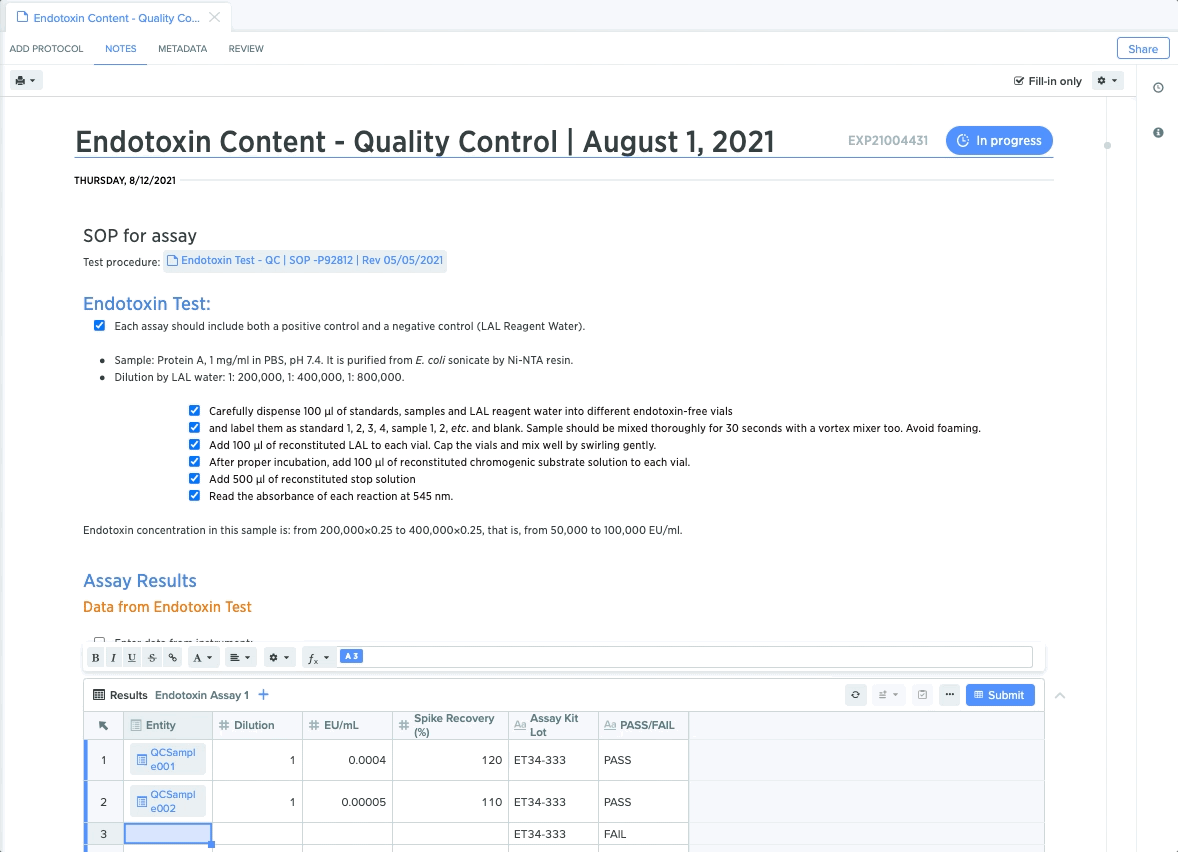
Quality Control: Maintain Compliant Sample Testing
For quality control teams, rigid protocols for sample testing and results capture are critical for maintaining end-to-end compliance. From protein detection to plasmid stability, each step requires teams to conduct specific tests and guide collaborators through test execution.
QC leads can create templates for each test, and lock down specific procedures to guide QC teams through the necessary steps. They can also embed structured data tables to guarantee accurate and compliant data capture. This makes it easier to trace QC test results back to sample for end-to-end traceability.
These new fill-in template capabilities build upon a foundation of rich functionality that unifies teams and data across R&D.
Powering breakthroughs for over 1,300 biotechnology companies, from startups to Fortune 500s
