How to avoid dauer-associated effects on longevity under reduced Insulin/IGF-1 signaling in the nematode Caenorhabditis elegans
Contributed by:
COLLIN YVÈS EWALD, Principal investigator at Eidgenössische Technische Hochschule
T. KEITH BLACKWELL, Principal investigator at Harvard Medical School
Editor’s Note: Understanding how and why we age has long puzzled scientists. One way to study this question and explore the molecular mechanisms mediating longevity is by using the nematode C. elegans as a model system. Collin Ewald, who recently started his own lab in the Department of Health Sciences and Technology in Zurich, Switzerland, and Keith Blackwell from Harvard Medical school spoke with Benchling about two new methods that can control for dauer-mediated processes when studying insulin signaling and longevity in C. elegans.
Benchling fosters collaboration and promotes new methodologies and standardization in science. If we could help share your research, let us know.
Introduction
The question of how and why we age has been a longstanding mystery. Scientists used to think that random wear and tear to the body caused aging. However, the emerging concept on aging now suggests that it is a dynamic process, influenced by our genetic background, environment, and numerous other factors. In fact, two groups of scientists led a breakthrough in the molecular investigation of aging more than 20 years ago and showed that a mutation in a single gene can double the lifespan of an organism [1-2].
Since then, scientists have identified several ways to slow aging -- termed longevity interventions. Some of these longevity interventions include reducing insulin/IGF-1 signaling, TOR signaling, protein translation, germ stem cell signaling and dietary intake [3]. However, we still do not understand why these interventions lead to longevity.
Use a nematode to understand the underlying mechanism of longevity interventions
To gain insight into how longevity interventions ensure a healthy long life, we set out to study how one of the most studied longevity interventions, reduced Insulin/IGF-1 signaling (rIIS), increases lifespan in the nematode Caenorhabditis elegans [4].
Using C. elegans provides several advantages:
C. eleganshas a short lifespan of about three weeks.
C. elegansis easy to cultivate. You can track genetic manipulation easily and its genes are well characterized.
Genes in C. elegans are highly conserved across species, including humans [5]. For example, reducing rIIS increases lifespan across species from C. elegansto fruit flies to mice. Notably, single nucleotide polymorphisms in the insulin/IGF-1 pathways have been associated with longevity in humans [6].
Separating dauer-related mechanisms from rIIS-mediated longevity in C. elegans
Although C. elegans provides a good system for identifying novel mechanisms that promote healthy aging under rIIS conditions, the C. elegans rIIS pathway also regulates a developmental arrest stage that can be activated improperly during adulthood [4,7]. This developmental arrest stage is called dauer, the German word for "endurance". Although dauer itself can contribute to longevity, when studying rIIS-mediated longevity in C. elegans, this arrest stage becomes problematic. Distinguishing between rIIS-mediated longevity and rIIS activated dauer effects is an important aspect to consider when identifying mechanisms behind rIIS longevity.
Understanding rIIS activated dauer
Under favorable conditions, C. elegans develop from eggs through four larval stages (L1-L4) within two to three days and in the laboratory show an adult lifespan of three weeks (Figure 1). By contrast, under harsh environmental conditions, C. elegans develops into dauer larvae that can survive for more than three months and withstand toxic insults and starvation (Figure 1).
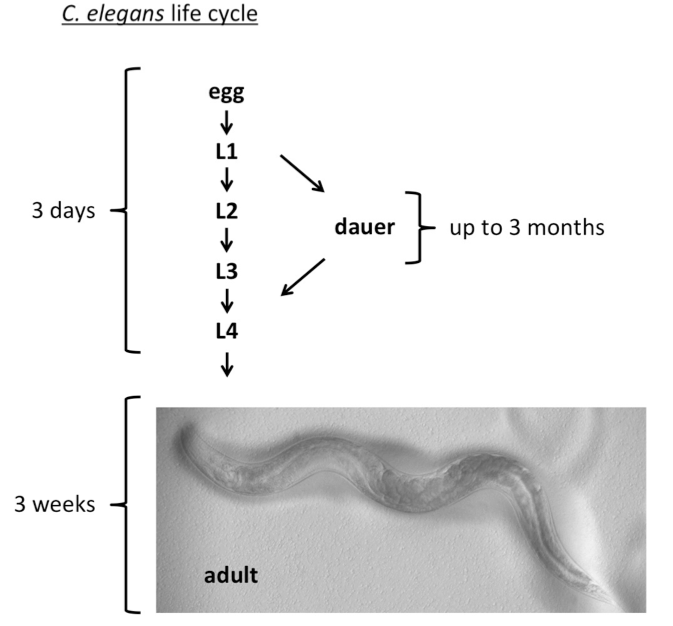
Figure 1.
During development, low insulin/IGF-1 signaling forces animals into dauer even when plenty of food is available [8]. In C. elegans, the insulin receptor (InsR) and the IGF-1 receptor (IGF-1R) is encoded by the single gene called daf-2 [9]. The daf-2pathway is one of the most studied genes/pathways in C. elegans with more than 1000 publications according to wormbase and 22 hypomorphic daf-2 alleles [10]. Mutations in daf-2 were isolated in genetic screens for mutants that develop into dauers at 25°C but develop into normal reproductive adults at 15°C. Interestingly, some of these daf-2mutants, when grown at higher temperatures (20-25°C), show dauer-like phenotypes (reduced movement, reduced brood-size, reduced body-size, abnormal gonad), suggesting that aspects of the dauer program are improperly activated during adulthood (dauer condition). By contrast, these same daf-2 mutants grown at 15°C look like wild type and do not display any dauer-like traits in adulthood (non-dauer condition) [7]. Importantly, daf-2 mutants are comparably long-lived across a broad temperature range (15-25°C) [4,7], suggesting that this improper activation of dauer-like traits can be uncoupled phenotypically.
These observations led us to investigate the genetic basis of these dauer-traits on rIIS longevity. Our recent work has shown that there are two genetically distinct pathways of how reduced insulin/IGF-1 signaling can increase longevity: a dauer-dependent and independent pathway (Ewald et al. 2015). This raises the issue of how to separate these two pathways to identify rIIS downstream mechanisms while controlling for dauer-related mechanisms.
Option 1. Performing longevity assays with daf-2mutants at 15°C
Environmental temperature can influence the C. elegans’ lifespan. The standard laboratory cultivating and assay temperature is 20°C. Wild-type C. elegans maintained at 15°C live longer, and wild type maintained at 25°C live shorter compared to those grown at 20°C. For the commonly used wild type C. elegans (N2), 25°C is already a stressful temperature. At 27°C, about 10% of the wild-type C. elegans develop into dauer larvae, indicating harsh environmental conditions. The classically used daf-2 mutants (e.g., daf-2(e1370)) do not form dauers at 15°C, form a few dauers at 20°C, and form all dauers at 25°C [7]. However, proportional to the temperature, the extension in lifespan of daf-2 mutants is the same [4,7]. Hence, performing the lifespan assay at 15°C avoids the activation of the dauer-associated pathway.
Pros
Avoids dauer-related traits during aging such as reduced movement, reduced body-size, and reduced brood size.
Cultivating temperature of 15°C facilitates harvesting L4 daf-2mutants for lifespan assay at a similar rate as wild-type C. elegans, since at 15°C daf-2mutants show no sterility, do not form dauers, and do not have a slowed developmental rate.
Cons
The lifespan assay takes longer.
Temperature-sensitive-alleles of other genes that need to be kept at 25°C cannot be used in combination with daf-2 alleles.
Option 2. Performing longevity assays with daf-2RNAi during adulthood
Depending on time of RNA interference, daf-2 may or may not be efficiently knocked-down. Knocking-down daf-2 by RNA interference (RNAi) starting on the first day of adulthood increases lifespan under all temperatures (15-25°C)[4]. However, applying daf-2(RNAi) during an earlier development stage does not lead to the formation of dauers [4,11] or the improper activation of the dauer-program during adulthood [4]. This is because neurons, which play a crucial role in the decision to enter the dauer arrest stage, are refractory to RNAi feeding and daf-2 is presumably not or less efficiently knocked-down in wild-type animals when RNAi is given during development [11].
Pros
Feeding C. elegans daf-2(RNAi)works during adulthood and avoids any contribution from developmental processes.
daf-2(RNAi)works in almost any genetic background. Therefore, there is no need to perform laborious crossing to other mutants.
Cons
RNAi bacteria are a different E. colistrain (HT115 instead of the normally used OP50). OP50 and HT115 have different nutritional content and affect the physiology of C. elegans. For example, wild-type C. eleganson HT115 has a slightly longer mean lifespan (~2 days) compared on OP50.
Summary
The nematode C. elegans provides advantages to identify mechanisms for prolonging health and lifespan under reduced insulin/IGF-1 conditions. The C. elegans rIIS controls both dauer development and longevity, and the improper activation of the dauer-associated program during adulthood can mask other distinct mechanisms that are mobilized under rIIS. The protocol options (1 and 2) outlined above provide methods for avoiding these dauer-associated contributions.
The way longevity experiments have been performed has broad implication on how we interpret lifespan assays and also mRNA expression profiling performed under conditions that activate the dauer program. Disentangling the dauer vs non-dauer contribution to rIIS longevity might help to reconcile the difference of differentially regulated downstream genes in daf-2 mutants.
Importantly, studying the mechanisms of rIIS longevity in C. elegans can provide information for more relevant species. Interestingly, treating aged mice with "C. elegans-dauer-pheromone" extended the mice's lifespan and attenuated several of their age-associated pathologies [12]. Hence, we speculate that each of these pathways (dauer or non-dauer) is likely to be of relevance to mammals, and it will be necessary to distinguish between them in order to understand their independent roles in mediating longevity.
To learn more about how we studied the mechanism of rIIS longevity, please see our full manuscript in Nature.
Additional resources
An extended discussion on dauers and non-dauer traits can be found in the supplement of (Ewald et al. 2015) under Supplementary Discussion p. 21. http://www.nature.com/nature/journal/v519/n7541/extref/nature14021-s1.pdf
A video showing the dauer-associated reduced motility during aging in daf-2(e1370) mutants.
daf-2(e1370)at 15°C: http://www.nature.com/nature/journal/v519/n7541/fig_tab/nature14021_SV1.htm
daf-2(e1370) at 20°C: http://www.nature.com/nature/journal/v519/n7541/fig_tab/nature14021_SV2.html
A protocol for performing lifespan assays with C. elegans can be found by this article written by Amrit et al. [13].
A good introduction to C. elegans is found in this reference written byCorsi et al [14].
Acknowledgment
We thank Rachel Beltzhoover for comments, Giulia Marthaler from ETH for making the headshot and Felix Marbet for making the lab logo.
Bibliography
Friedman, D.B. & Johnson, T.E., 1988. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics, 118(1), pp.75–86.
Kenyon, C.J. et al., 1993. A C. elegansmutant that lives twice as long as wild type. Nature, 366(6454), pp.461–464.
Kenyon, C.J., 2010. The genetics of ageing. Nature, 464(7288), pp.504–512.
Ewald, C.Y. et al., 2015. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature, 519(7541), pp.97–101.
Shaye, D.D. & Greenwald, I., 2011. OrthoList: a compendium of C. elegansgenes with human orthologs. K. M. Iijima, ed. PloS one, 6(5), p.e20085.
Ziv, E. & Hu, D., 2011. Genetic variation in insulin/IGF-1 signaling pathways and longevity. Ageing research reviews, 10(2), pp.201–204.
Gems, D. et al., 1998. Two pleiotropic classes of daf-2mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics, 150(1), pp.129–155.
Riddle, D.L., Swanson, M.M. & Albert, P.S., 1981. Interacting genes in nematode dauer larva formation. Nature, 290(5808), pp.668–671.
Kimura, K.D. et al., 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science (New York, NY), 277(5328), pp.942–946.
Patel, D.S. et al., 2008. Clustering of Genetically Defined Allele Classes in the Caenorhabditis elegans DAF-2 Insulin/IGF-1 Receptor. Genetics, 178(2), pp.931–946.
Kennedy, S., Wang, D. & Ruvkun, G., 2004. A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans.Nature, 427(6975), pp.645–649.
Park, J.H. et al., 2014. Daumone fed late in life improves survival and reduces hepatic inflammation and fibrosis in mice. Aging cell, pp.n/a–n/a.
Amrit, F.R.G. et al., 2014. The C. elegans lifespan assay toolkit. Methods (San Diego, Calif), 68(3), pp.465–475.
Corsi, A.K., Wightman, B. & Chalfie, M., 2015. A Transparent Window into Biology: A Primer on Caenorhabditis elegans.Genetics, 200(2), pp.387–407.
Powering breakthroughs for over 1,300 biotechnology companies, from startups to Fortune 500s
