Modernize your lab informatics for the next generation of antibody and protein R&D
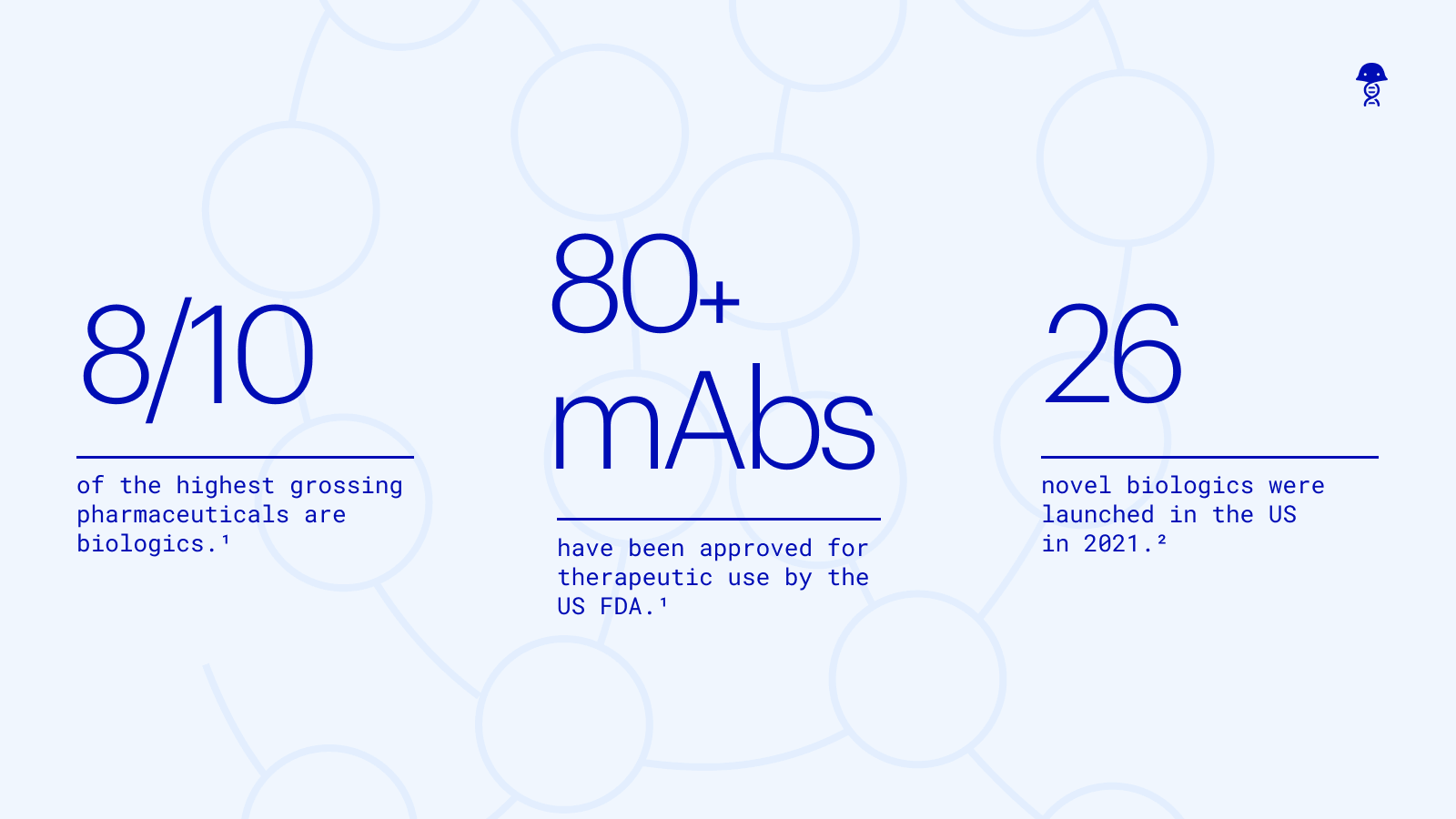
It’s been over 35 years since the first monoclonal antibody was cleared by the FDA. In that time, the biotechnology industry has developed increasingly sophisticated ways to discover, develop, and manufacture innovative biologics. Eight of the top ten medications are biologics, a telling sign of their therapeutic potential. But unmet medical needs still persist, and the industry has responded with new approaches such as bispecifics, antibody-drug conjugates, and immunocytokines. As the complexity and variance of these emerging modalities increases, so too does the need for software that can support this next generation of biologics R&D.
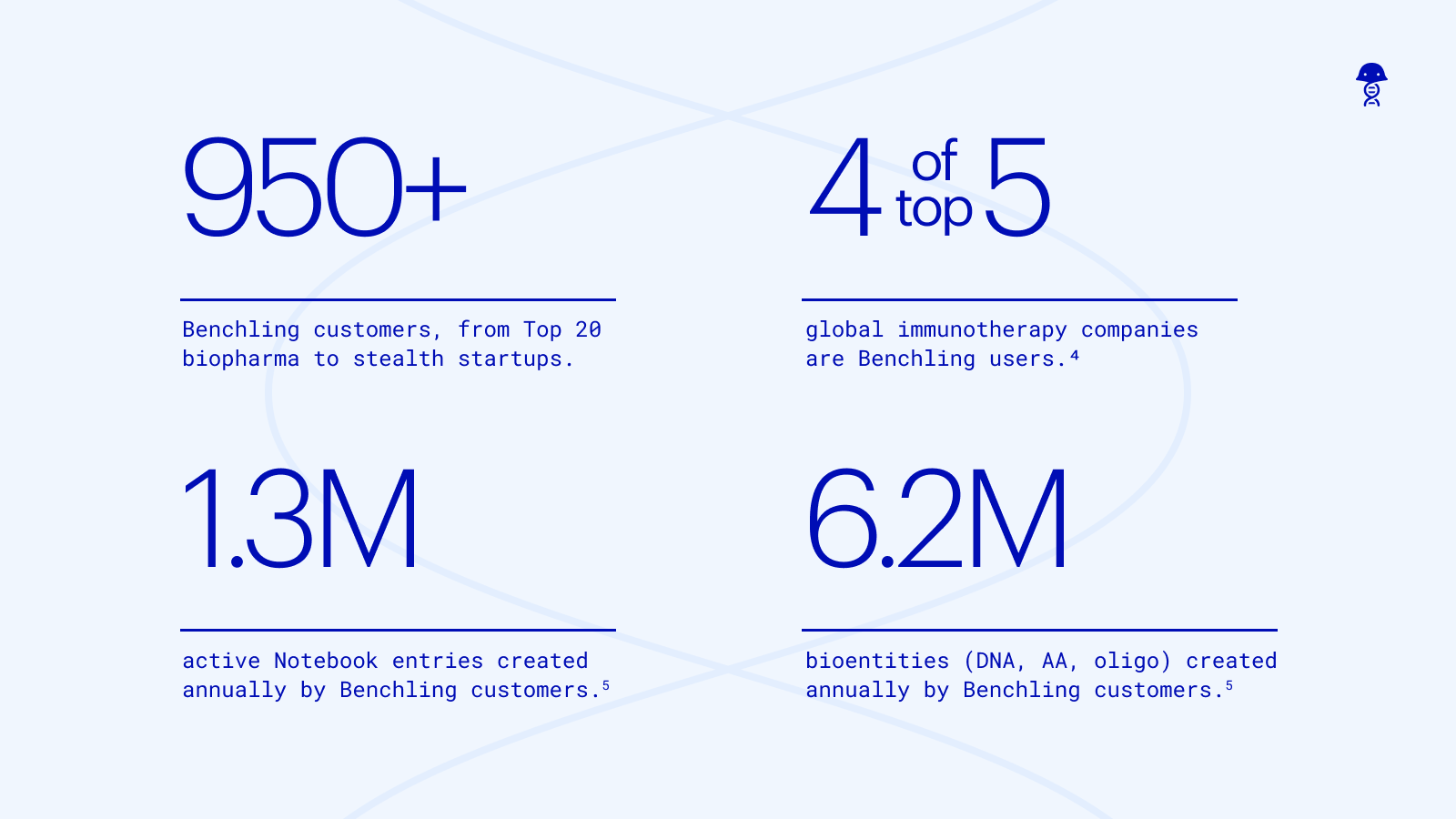
Here, we’ve captured some of the key observations made from working with 250+ innovators in the field of antibodies and protein R&D.
Antibody and protein therapeutics are on the rise (again)
It’s been 35 years since the first antibody therapeutic was approved. This class of drug compounds continues to show promise towards treating a range of disease conditions with greater specificity and fewer adverse effects.
New protein engineering technologies are giving rise to new antibody mechanisms of action
Antibodies can operate directly on disease targets to induce a therapeutic effect, such as apoptosis of cancer cells or using immune checkpoint inhibitors. Newer advances in the field have made strides in increasing target specificity and improving the overall therapeutic payload of the drug.
Immunocytokines
antibody-drug conjugate
antibody-radionuclide conjugates
bispecific antibodies
immunoliposomes
chimeric antigen receptor T cell (CAR-T) therapy

The pace of innovation required to stay competitive continues to accelerate
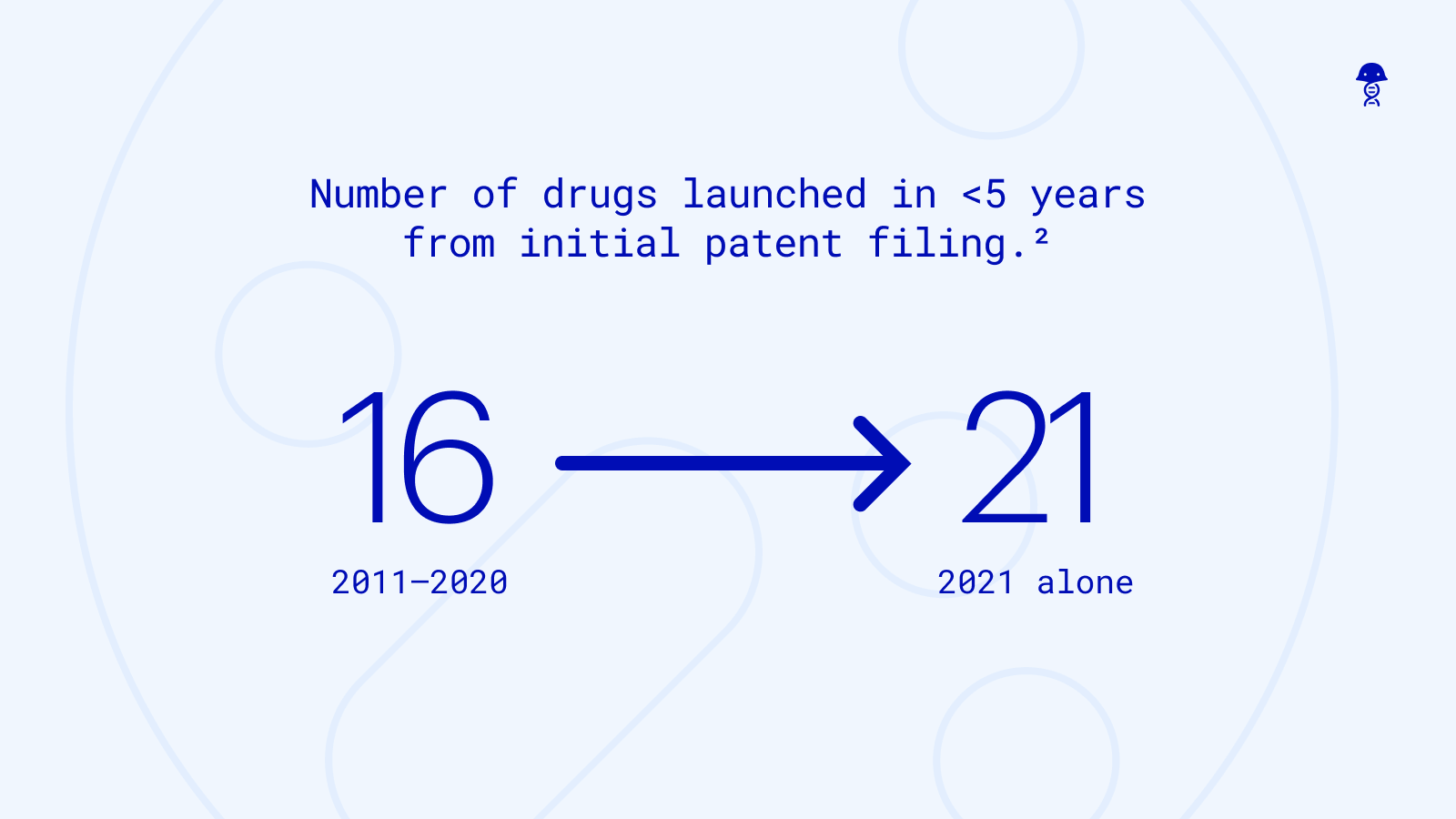
Biopharmaceutical companies are bringing novel biologics to market faster than ever before. The median time to bring a novel active substance (NAS) from initial patent filing to market introduction has accelerated from over 15+ years in 2016 down to 11 years in 2021, driven by investments in R&D productivity, clinical innovation, and expedited review pathways. While bringing a drug to market within 5 years of patent filing used to be a rare occurrence, it is now far more common.

Biggest obstacles when trying to adopt legacy informatics software for antibody and protein R&D
| Not designed for biology | Designed for small molecule and lacks biological awareness. |
|---|---|
| Unstructured data | Lack of organized and uniform relationships across data makes it difficult to search for and analyze results. |
| Rigid and brittle implementations | Software that is difficult to update and evolve reduces effectiveness in supporting R&D. |
| Disconnected applications | Separate tools for molecular design, documentation, and sample management creates process inefficiency. |
| Doesn’t support collaboration | Tools don’t reflect the needs of modern, specialized teams that work together with frequent handoffs. |
Top priorities to consider when aligning your informatics to antibody R&D workflows
| Focus areas | R&D value |
|---|---|
| Bring antibody design and sequence analysis tools onto common platform with other critical functions, such as experimental documentation and inventory management. | Simplify user experience Establish connectivity across applications and databases |
| Ensure that all antibody R&D teams have collaboration tools to share data and make routine sample and workflow transfers. | Accelerate scientific workflows Streamline collaboration and hand-offs |
| Select informatics solutions that can span the Research and Development functions, going from more exploratory and design work though to antibody characterization and preclinical evaluation. | Reduce IT cost and complexity Establish a scalable, high-performing IT solution across R&D |
| Anticipate evolution of your antibody R&D and select informatics tools that can readily be configured with updates to data models, experimental templates, and results capture. | Improve scientist productivity Improve IT agility to adapt to changes in workflows |
Join a community of Benchling users committed to advancing biotechnology
“Benchling offers a very simple way of naming molecules and storing the sequences... it's [now] in a format where we can learn more complex lessons from our historical data. In that sense, managing data in a more structured, formatted way is very powerful.”
References
Lu, RM., Hwang, YC., Liu, IJ. et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 27, 1 (2020). https://doi.org/10.1186/s12929-019-0592-z
Global Trends in R&D: Overview through 2021. IQVIA, February 2022
Fu, Z., Li, S., Han, S. et al. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Sig Transduct Target Ther 7, 93 (2022). https://doi.org/10.1038/s41392-022-00947-7
https://www.biospace.com/article/global-top-10-cancer-antibodies-by-sales-2021/
Benchling internal product utilization data, accessed June 2022